
-
 19PSFM 物理源性醫用無血清幹細胞強化培養基套組國立中山大學許晉銓教授發明人:許晉銓/顏育達領域:醫療器材適應症:幹細胞培養研發階段:醫材雛型開發Prototype development摘要:
19PSFM 物理源性醫用無血清幹細胞強化培養基套組國立中山大學許晉銓教授發明人:許晉銓/顏育達領域:醫療器材適應症:幹細胞培養研發階段:醫材雛型開發Prototype development摘要:再生醫學的技術不斷進步,目前已可將幹細胞應用於多種疾病的治療、新藥開發及組織器官列印,全球幹細胞應用市場預估在2030年躍昇至2610億美元,而這些幹細胞的技術應用均需使用細胞培養放大的過程。本團隊歷經十年研究探索基因調控對於細胞複製增生及分化能力的影響,開發出基於物理源性的幹細胞強化無血清培養產品組,有別於一般產品著重於化學性刺激改變細胞本質(如ligand-receptor axis)對細胞幹化性的影響,本技術產品主要是藉由探討化合物組合進行物理性刺激如何有效且穩定地誘導細胞幹化性的進程,進而達到強化幹細胞增生複製及分化的結果,並透過醫療等級的認證佈局未來用於輔助目前細胞治療的臨床應用,本產品在發展的策略與思維上具有極高的新穎性與差異性
Regenerative medicine applications are becoming increasingly widespread. Currently, stem cells can be used in the treatment of various diseases, drug development, and the 3D bioprinting of organs. The global stem cell market is estimated to reach $261 billion by the year 2030. Most stem cell technologies require a process of cell culture amplification for their applications. After a decade of research on the impact of gene regulation on cell replication and differentiation capabilities, our team has developed a serum-free cell culture product line - PSFM that enhances stem cell capabilities through physical characteristics. Differing from conventional products that mainly focus on altering cellular properties through chemical stimuli for cell activation, PSFM primarily relies on a combination of compounds to provide effective and stable induction of cellular activation through physical stimuli. Ultimately, this leads to enhanced stem cell replication and differentiation. Our next step is to obtain a Class II medical device certification for the product, allowing PSFM to be used in clinical treatments in the future. Compared to past technologies and products, PSFM possesses an exceptionally high level of novelty and distinctiveness.
-
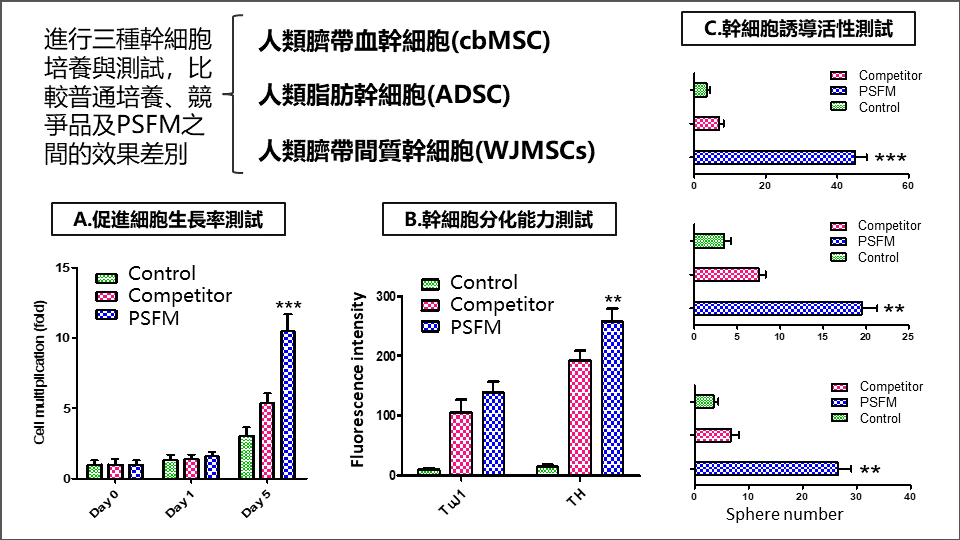
進行細胞培養與測試,比較普通培養、競爭品及團隊產品之間的各種指標效果差別
 20居家呼吸監測與互動式肺復原臺北醫學大學.雙和醫院重症科曾健華副教授.主任發明人:曾健華領域:醫療器材適應症:BuddyRT適用於患者居家使用,以監測PEF(峰值呼氣流量)和FEV1(一秒鐘內的強制呼氣量),並可引導患者做吐氣正壓治療、吸氣肌、吐氣肌之肺復原訓練。研發階段:申請試驗用藥物/新醫材(試劑) Investigational New Drug (IND)/ Investigational Device Exemption (IDE) application摘要:
20居家呼吸監測與互動式肺復原臺北醫學大學.雙和醫院重症科曾健華副教授.主任發明人:曾健華領域:醫療器材適應症:BuddyRT適用於患者居家使用,以監測PEF(峰值呼氣流量)和FEV1(一秒鐘內的強制呼氣量),並可引導患者做吐氣正壓治療、吸氣肌、吐氣肌之肺復原訓練。研發階段:申請試驗用藥物/新醫材(試劑) Investigational New Drug (IND)/ Investigational Device Exemption (IDE) application摘要:當前全球面臨人口老化、空氣污染、傳染病擴散等問題,使呼吸系統疾病的醫療需求持續上升。根據WHO報告,全球前六大死因中有三個是與肺部疾病有關,每年約有650萬人死於此,數字持續增長。特別是肺阻塞患者由於缺乏自我監測工具和肺復原支持,容易急性惡化,增加急診和重症醫療的負擔。COVID-19疫情爆發後,居家照護趨勢明顯。研究顯示,每日監測呼氣流速可提前3天警示呼吸道疾病惡化,規律肺復原可減少急診次數,降低62.7%死亡率,正確吸藥也可減低57.2%死亡率。
我們團隊在雙和醫院擁有10年以上的呼吸道照護和肺復原經驗,將照護概念融入資訊系統,開發了呼吸道疾病患者個案管理和復原照護平台,並獲得認證。為擴展高品質照護至居家,我們開發了可攜式呼吸流量監測設備和互動式呼吸阻力訓練器(BuddyRT®),已經獲得多項獎項。我們目前正籌備量產,並進行驗證,以確保實證成效。
明年計劃包括取得TFDA販售許可證,開發互動APP和遠程照護平台,以及進一步的臨床驗證。我們短期目標是在2024-2025年間在台灣市場推出,中期目標是獲得FDA/CE認證,長期目標是進入美國和歐盟市場。我們預期這項技術將提供呼吸疾病患者遠程照護,減少急性惡化和死亡率,同時減輕社會福利負擔。The world is currently facing challenges such as an aging population, air pollution, and the spread of infectious diseases, leading to a continuous increase in the demand for respiratory healthcare. According to a WHO report, three out of the top six global causes of death are associated with pulmonary diseases, resulting in approximately 6.5 million annual deaths, with numbers steadily rising. Particularly, patients with obstructive lung diseases are susceptible to acute exacerbations due to the lack of self-monitoring tools and support for pulmonary rehabilitation, which places a burden on emergency and critical care. The trend towards home-based care has become more pronounced following the outbreak of the COVID-19 pandemic.
Research shows that daily monitoring of peak expiratory flow can provide a three-day early warning of respiratory disease deterioration. Regular adherence to pulmonary rehabilitation can reduce emergency room visits by 62.7%, and proper medication adherence can lower mortality rates by 57.2%.
Our team has accumulated over a decade of experience in respiratory care and pulmonary rehabilitation at Shuang Ho Hospital. We have integrated the concept of care into information systems and developed a platform for individualized management and rehabilitation care for respiratory disease patients, receiving certification. To extend high-quality care to homes and improve self-monitoring and adherence to pulmonary rehabilitation, we have developed a portable dual-function respiratory flow monitoring device and an interactive respiratory resistance training device (BuddyRT®), both of which have received multiple awards. Currently, we are preparing for mass production and conducting validation to ensure evidence-based efficacy.
Our future plans include obtaining certification, developing an interactive app and remote care platform, and further clinical validation. Our short-term goal is to launch in the Taiwan market with TFDA certification by 2024-2025, secure angel funding for stability, and obtain FDA/CE certification in the mid-term. In the long-term, we aim to enter the US and European markets, conducting multinational clinical trials. We anticipate that this technology will provide respiratory disease patients with remote care, reducing acute exacerbations and mortality rates, while alleviating the burden on social welfare.-
我們開發了一款二合一可攜式呼吸流量監測裝置和互動式呼吸阻力訓練器(BuddyRT®),除了讓病患居家正確測量肺功能,還可讓病患依據肺功能情形調整訓練阻力。
 21積層製造3D生物陶瓷骨移植替代物高雄醫學大學醫藥暨應用化學系王志光教授發明人:王志光教授 等人領域:醫療器材適應症:生物陶瓷骨移植替代物研發階段:候選藥物/醫材雛型臨床前試驗 Preclinical trials摘要:
21積層製造3D生物陶瓷骨移植替代物高雄醫學大學醫藥暨應用化學系王志光教授發明人:王志光教授 等人領域:醫療器材適應症:生物陶瓷骨移植替代物研發階段:候選藥物/醫材雛型臨床前試驗 Preclinical trials摘要:本團隊利用了光固化暨負溫感水膠與生物陶瓷專利陶瓷漿料系統搭配3D列印技術,開發出一種革命性的仿生骨植入物。能夠製造具有精緻性、複雜度及抗壓強度的產品。透過光固化生坯成型後,在高溫燒結過程中,負溫感水膠聚合,促進燒結緻密化,進一步增強產品的抗壓強度。這項技術有望成為醫療界的革命性突破,提高骨植入物的效能。
Our team has developed a revolutionary bionic bone implant using a light-curing, negative temperature-sensitive hydrogel and a patented ceramic slurry system for bioceramics with 3D printing technology. It is capable of producing products with refinement, complexity and compressive strength. After light-curing the raw material, the sintering process is carried out at high temperature and the negative temperature-sensitive hydrogel is polymerized to promote sintering densification and further enhance the compressive strength of the product. This technology is expected to be a revolutionary breakthrough in the medical field, improving the performance of bone implants.
-
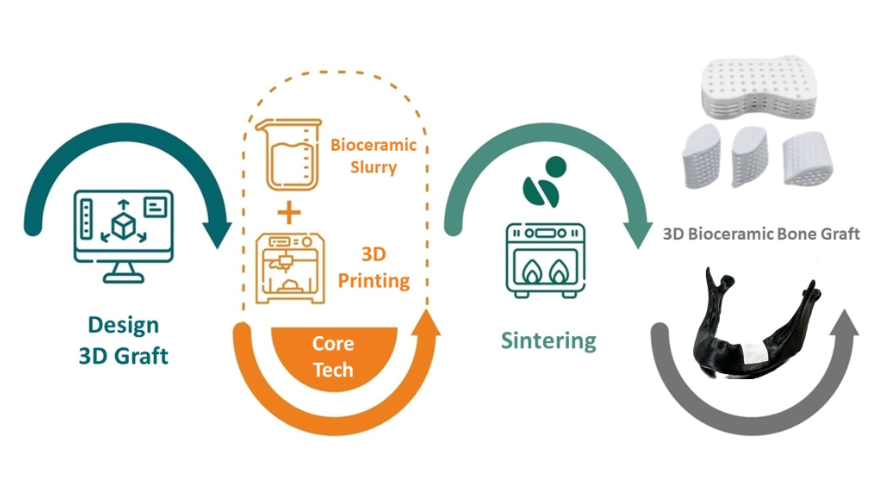
積層製造3D生物陶瓷骨移植替代物製程流程圖
 22胸腔深度學習:人工智慧多模影像精準健康平台臺北醫學大學/醫學系放射學科陳震宇教授發明人:陳震宇領域:醫療器材適應症:肺部結節/心臟冠狀動脈鈣化/骨質疏鬆研發階段:查驗型臨床試驗摘要:
22胸腔深度學習:人工智慧多模影像精準健康平台臺北醫學大學/醫學系放射學科陳震宇教授發明人:陳震宇領域:醫療器材適應症:肺部結節/心臟冠狀動脈鈣化/骨質疏鬆研發階段:查驗型臨床試驗摘要:臺北醫學大學最新成立衍生新創公司「神瑞人工智慧」,致力於運用人工智慧推動智慧醫療。本團隊由資深臨床醫師引領,並導入實際臨床流程開發出Deep-Lung。利用單一低劑量胸腔電腦斷層影像LDCT,使用多模人工智慧技術,將肺臟,心臟與胸椎骨做一次性的篩檢,自動偵測肺節結、自動計算冠狀動脈鈣化分數、和自動定位量測骨質密度,讓肉眼不易看到的疾病訊息可以被看到,且只要點擊一下就能自動產出標準化的文字報告,給予醫師參考。團隊的研發成果屢創佳績,Deep-Lung,Lung cancer DCSS決策系統以及Deep Brain曾獲多次國家新創獎及兩項未來科技獎(InnoAward及Futex),並獲多家媒體報導。神瑞人工智慧的目標是通過影像篩檢產品為年長者提供早期檢測肺癌、冠心病、骨質疏鬆症和失智症等疾病的高效解決方案。
Taipei Medical University founded a newest spin-off startup company DeepRad.AI, with a focus on leveraging AI to realize smart healthcare.Led by experienced radiologists, DeepRad.AI has developed Deep-Lung and successfully integrated it into the real-world clinical workflow. Utilizing low-dose computed tomography (LDCT) and employing multi-model artificial intelligence technologies, Deep-Lung enables simultaneous screening of the lung, heart, and spine in a single examination. It automatically detects lung nodules, predicts coronary artery calcium (CAC) scores, and measure bone mineral density. Moreover, Deep-Lung provides end-to-end solution from imaging screen to auto-reports for radiologists by just one click. The team's research and inventions, including Deep-Lung and other products such as DeepBrain and lung cancer decision support system, have been recognized and granted prestigious national awards such as InnoAward and Futex, and have also gained several media exposures. The aim of DeepRad.AI is to provide efficient solutions for the early detection of lung cancer, coronary disease, osteoporosis and dementia among the aging population through imaging screening products.
-

研發團隊成員
 23金屬有機框架應用於皮膚纖維化治療國立臺灣大學化學工程學系/國家衛生研究院醫奈所吳嘉文特聘教授/所長發明人:吳嘉文/高煌凱/鄭博修/鍾偉庭/翁在萱領域:新穎藥物適應症:蟹足腫及其他皮膚纖維化疾病研發階段:臨床檢體/細胞驗證 Clinical specimen/In vitro validation摘要:
23金屬有機框架應用於皮膚纖維化治療國立臺灣大學化學工程學系/國家衛生研究院醫奈所吳嘉文特聘教授/所長發明人:吳嘉文/高煌凱/鄭博修/鍾偉庭/翁在萱領域:新穎藥物適應症:蟹足腫及其他皮膚纖維化疾病研發階段:臨床檢體/細胞驗證 Clinical specimen/In vitro validation摘要:增生性疤痕與蟹足腫疤痕在目前臨床治療中沒有有效的單一治療,臨床上大多需進行多重合併治療來降低蟹足腫的復發率,然而對於患者所花費的時間與金錢成本非常高,且仍有機會復發,而本案我們我們主要合成以鐵為基礎之金屬有機框架(MIL-100(Fe)),並將MIL-100(Fe)與微針系統結合,以貼布形式貼附於患者欲治療部位。本案緣起主要由合法藥品去鐵胺(Deferoxamine, DFO),其在研究應用上已被證實可促進傷口癒合,DFO 可以提高傷口形成時細胞所需要的缺氧誘導因子(HIF-1α),然而在蟹足腫纖維母細胞中HIF-1α以及TGF-β1 所誘導的膠原蛋白I 型(collagen I)與平滑肌肌動蛋白(SMA)會過度表現,而本案之MIL-100(Fe)在研究結果顯示可降低蟹足種纖維母細胞的HIF-1α與TGF-β1,也大幅降低collagen I與SMA的表現,藉此達到治療蟹足腫的效果。
-
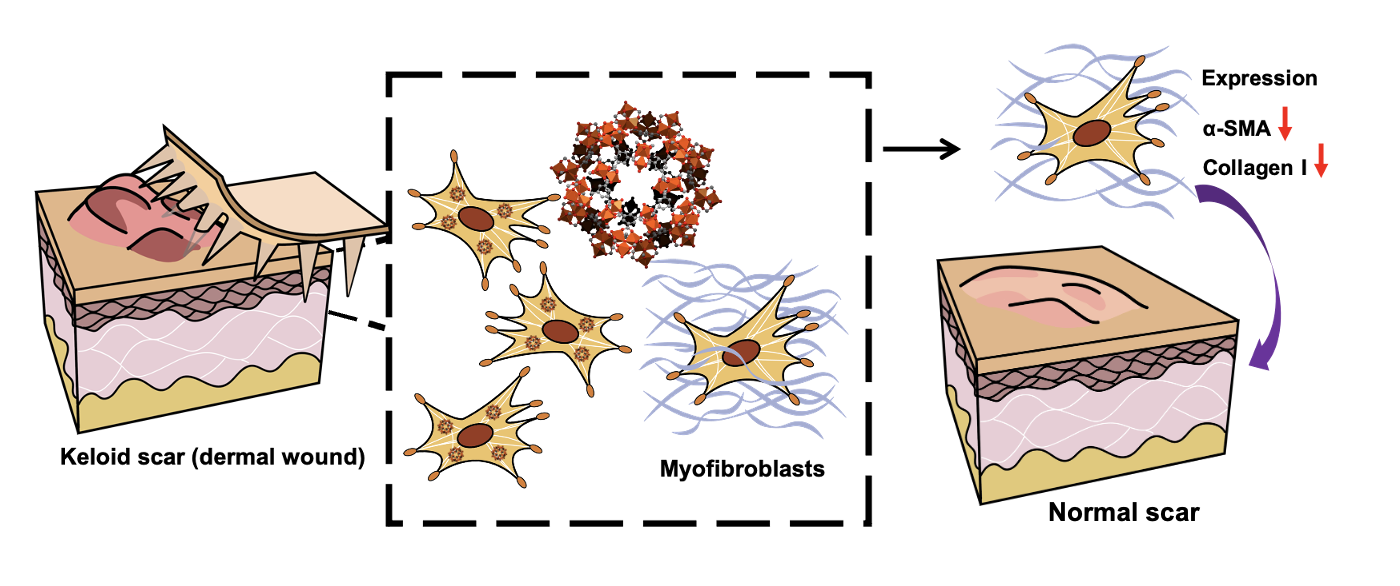
透過含有MIL-100(Fe)的微針貼片,將MIL-100(Fe)送進病灶內治療,蟹足腫纖維母細胞會進行細胞吞噬,進入纖維母細胞後的MIL-100(Fe)最終降低collagen I與SMA的表現,藉此達到治療蟹足腫的效果。
 24研發仿生腦模擬器應用於神經外科手術訓練國防醫學院醫學系劉偉修教授發明人:劉偉修/陳品銓領域:醫療器材適應症:神經外科手術研發階段:醫材雛型開發Prototype development摘要:
24研發仿生腦模擬器應用於神經外科手術訓練國防醫學院醫學系劉偉修教授發明人:劉偉修/陳品銓領域:醫療器材適應症:神經外科手術研發階段:醫材雛型開發Prototype development摘要:本研究利用3D列印、模具設計、彈性材料澆注技術,開發3D腦部手術訓練擬真模擬系統,做為訓練夾閉腦血管瘤手術及移除腦瘤手術的模擬器,此模擬器包括頭顱、大腦軟組織、腦室、腦膜、以及一管壁厚度可控制、全透明的威利氏環腦血管脈管系統,並在發生機率較高的血管區段製做腦血管瘤及大腦軟組織做腦瘤,做為訓練學員的教具。研究重點也針對血管壁材料做研發,讓此醫療模型具有類真實血管的觸感以及彈性,大幅提升模擬系統的真實度。訓練課程中將模擬血液打入腦血管,學員們根據不同位置的腦血管瘤、腦瘤,執行開顱、切開大腦軟組織、目視威利氏環腦血管再定位到腦血管瘤或是腦瘤、最後利用血管夾夾閉腦血管瘤或是移除腦瘤,完成整個訓練。
-
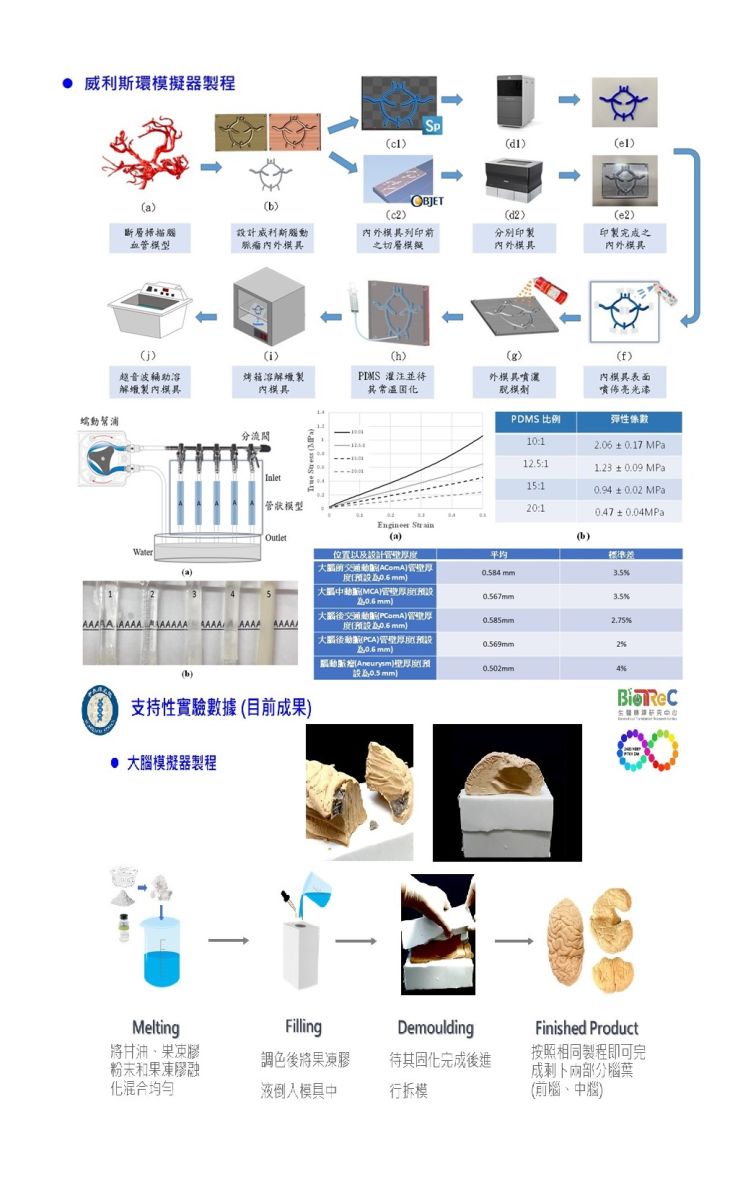
利用3D列印、模具設計、彈性材料澆注技術,開發3D腦部手術訓練擬真模擬系統,做為訓練夾閉腦血管瘤手術及移除腦瘤手術的模擬器,此模擬器包括頭顱、大腦軟組織、腦室、腦膜、以及一管壁厚度可控制、全透明的威利氏環腦血管脈管系統,並在發生機率較高的血管區段製做腦血管瘤及大腦軟組織做腦瘤,做為訓練學員的教具。
 25多功能智慧肺適能儀國防醫學院朱修儁呼吸治療師發明人:朱修儁領域:醫療器材適應症:重症與長照居家肺復能患者的AI檢測與訓練器材研發階段:醫材雛型開發Prototype development摘要:
25多功能智慧肺適能儀國防醫學院朱修儁呼吸治療師發明人:朱修儁領域:醫療器材適應症:重症與長照居家肺復能患者的AI檢測與訓練器材研發階段:醫材雛型開發Prototype development摘要:在呼吸治療重症到長照領域中,在檢查用途為呼吸器脫離指標(Weaning parameter);在訓練用途多以誘發性肺量計(Incentive spirometer) 以及呼吸肌肉訓練(Respiratory muscle training)為主。在COVID-19之後,居家與社區呼吸復健更為興盛,國外也開始在健身房進行呼吸復原活動,未來10年將增加600萬名65歲長者,在醫療人力缺乏之下,顯示個人化智慧醫療產品更為重要,本產品將感測器與APP結合,目標將開發自動記錄與AI建議訓練產品,未來將取代臨床醫療人員的衛教與使用監測。以目前健保資料庫計算,每年其總價值可達31億點,若可研發成功將可取代多樣產品,減少臨床人力之需求。
In the field of respiratory therapy, from critical care to long-term care, various parameters are used for assessment. For weaning purposes, the respiratory weaning parameter is employed. Training primarily focuses on the use of incentive spirometry and respiratory muscle training. Post-COVID-19, there has been a surge in home and community-based respiratory rehabilitation. Internationally, there is a growing trend of respiratory rehabilitation activities in fitness centers. Over the next 10 years, the population of seniors aged 65 and above is expected to increase by 6 million. Given the shortage of healthcare professionals, personalized smart healthcare products become even more crucial.
Our product combines sensors with a mobile app, aiming to develop an automated recording and AI-based training recommendation system. This innovation is poised to replace traditional clinical education and monitoring conducted by healthcare personnel. According to current healthcare database calculations, the annual market value of this product could reach 3.1 billion points. If successfully developed, it has the potential to replace various products and reduce the demand for clinical manpower.-


海報兩篇為軟體與硬體的研究展示,為2022年呼吸治療全國聯合會研討會海報
 26高通量、免標定奈米電漿子影像技術之發展與應用中央研究院生醫轉譯研究中心李光立專案研究員發明人:魏培坤、李光立領域:醫療器材適應症:細胞活力檢測研發階段:醫材雛型開發Prototype development摘要:
26高通量、免標定奈米電漿子影像技術之發展與應用中央研究院生醫轉譯研究中心李光立專案研究員發明人:魏培坤、李光立領域:醫療器材適應症:細胞活力檢測研發階段:醫材雛型開發Prototype development摘要:細胞活力檢測在生物醫學研究和藥物開發中具有重要性和廣泛的應用,例如,應用於研究細胞生理和疾病機制、藥物篩選和研發、毒性測試和環境評估、基因和蛋白質研究及腫瘤學研究。大多數的細胞活力檢測方法屬於標定式並讀取染色、螢光或冷光訊號的單一終點測定法。這些方法有一些限制,包括它們需要耗費大量勞力,除了裂解或固定步驟外還需標定處理。此外,這些測試對於藥物與目標細胞相互作用機制提供的資訊有限。團隊提出免標定、無毒性、即時、簡單、快速及高靈敏度的感測技術應用於貼附型細胞活性研究。
Comparative results of cytotoxicity tests with CCK-8 kit and SPR sensor testing.(a) The results indicate a consistent trend in cell viability and cell adhesion ratio with varying Doxorubicin concentrations, with an approximate half-inhibitory concentration of 4μM. (b) Images of CL1-0 cells after overnight incubation with varying Doxorubicin concentrations.
 27高效生殖細胞培養液高雄榮民總醫院李佳榮副研究員發明人:李佳榮領域:輔助性材料適應症:人工生殖體外細胞培養研發階段:候選藥物/醫材雛型臨床前試驗 Preclinical trials摘要:
27高效生殖細胞培養液高雄榮民總醫院李佳榮副研究員發明人:李佳榮領域:輔助性材料適應症:人工生殖體外細胞培養研發階段:候選藥物/醫材雛型臨床前試驗 Preclinical trials摘要:台灣少子化問題日益嚴重,隨年齡增長,女性卵子的質量會不可逆轉地下降,導致生育能力下降,同時也增加了胚胎品質下降、流產風險升高。研究發現,生殖老化影響生殖細胞品質,還降低女性生育率。為改善此問題,我們開發一種高效的體外生育培養液,其中包括維生素C和ROCK抑制劑(Y-27632)。維生素C是強效的抗氧化劑,有助於減少氧化壓力,保護細胞不受損害。ROCK抑制劑Y-27632則有助提升細胞品質。經過系統性篩選,結果發現使用高效培養液的細胞能降低活性氧(ROS)生成,並重建細胞粒線體功能,有助改善細胞品質。這對提高體外受精的成功率,尤其對高齡女性而言,具有重要意義。這項研究為不孕患者提供了更有效的治療途徑,並對人工生育領域產生積極影響。
The problem of declining birth rates in Taiwan is becoming increasingly severe. With age, the quality of women's eggs irreversibly decreases, leading to reduced fertility, increased risks of embryo quality degradation, and higher chances of miscarriages. Research has found that reproductive aging not only affects the quality of reproductive cells but also decreases women's fertility rates. To address this issue, we have developed an efficient in vitro fertilization culture medium, including vitamin C and a ROCK inhibitor (Y-27632). Vitamin C acts as a potent antioxidant, reducing oxidative stress and protecting cells from damage. The ROCK inhibitor Y-27632 contributes to enhancing cell quality. Through systematic screening, the results have shown that cells cultured using the high-efficiency medium can reduce the generation of reactive oxygen species (ROS) and restore mitochondrial function, thereby improving cell quality. This is of great significance in increasing the success rate of in vitro fertilization, particularly for older women. This research provides a more effective treatment approach for infertility patients and has a positive impact on the field of assisted reproduction.
-
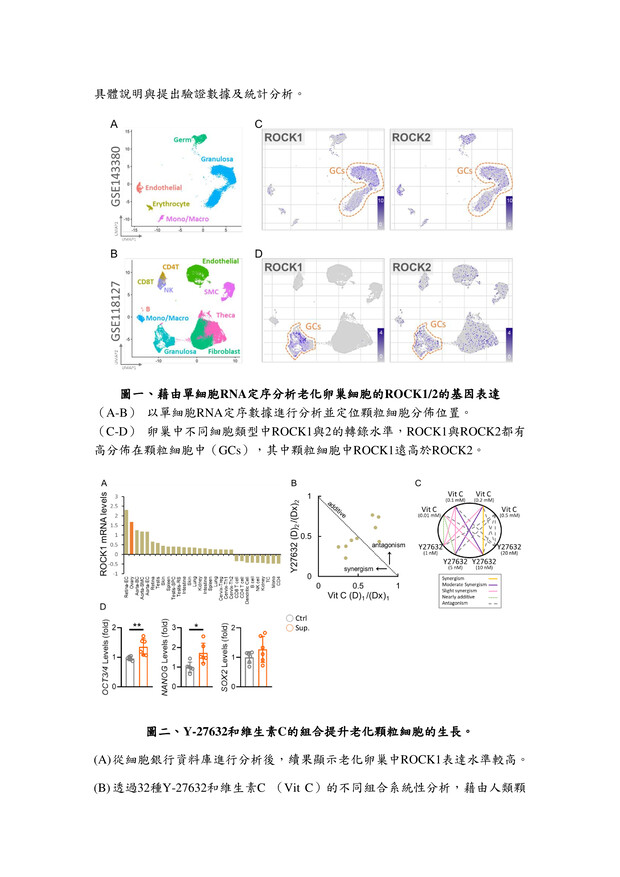
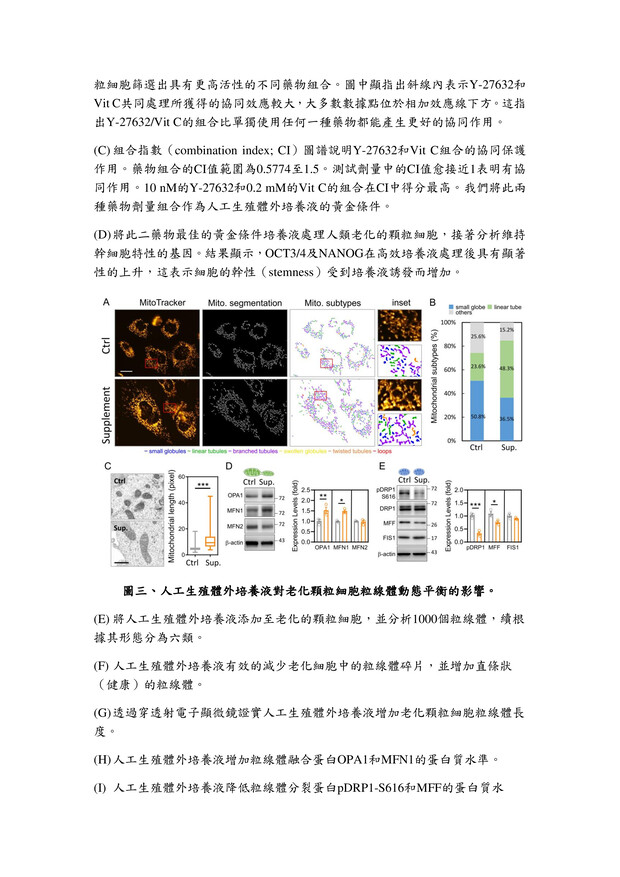
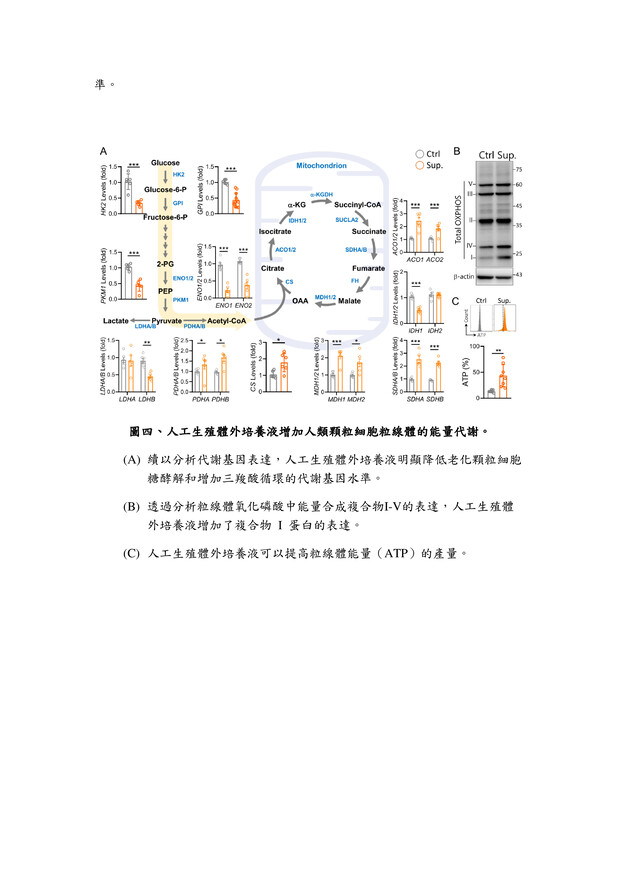
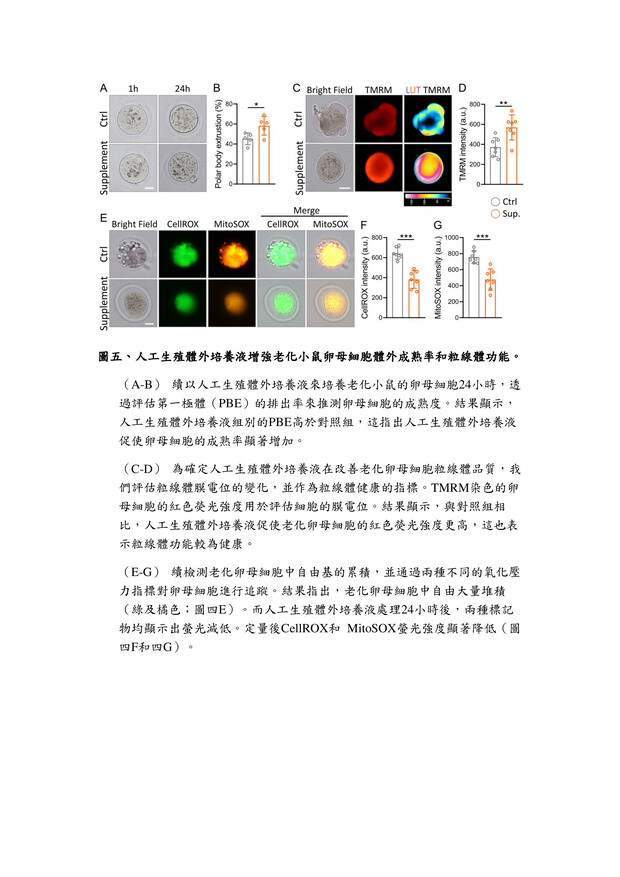
說明已附在圖表中
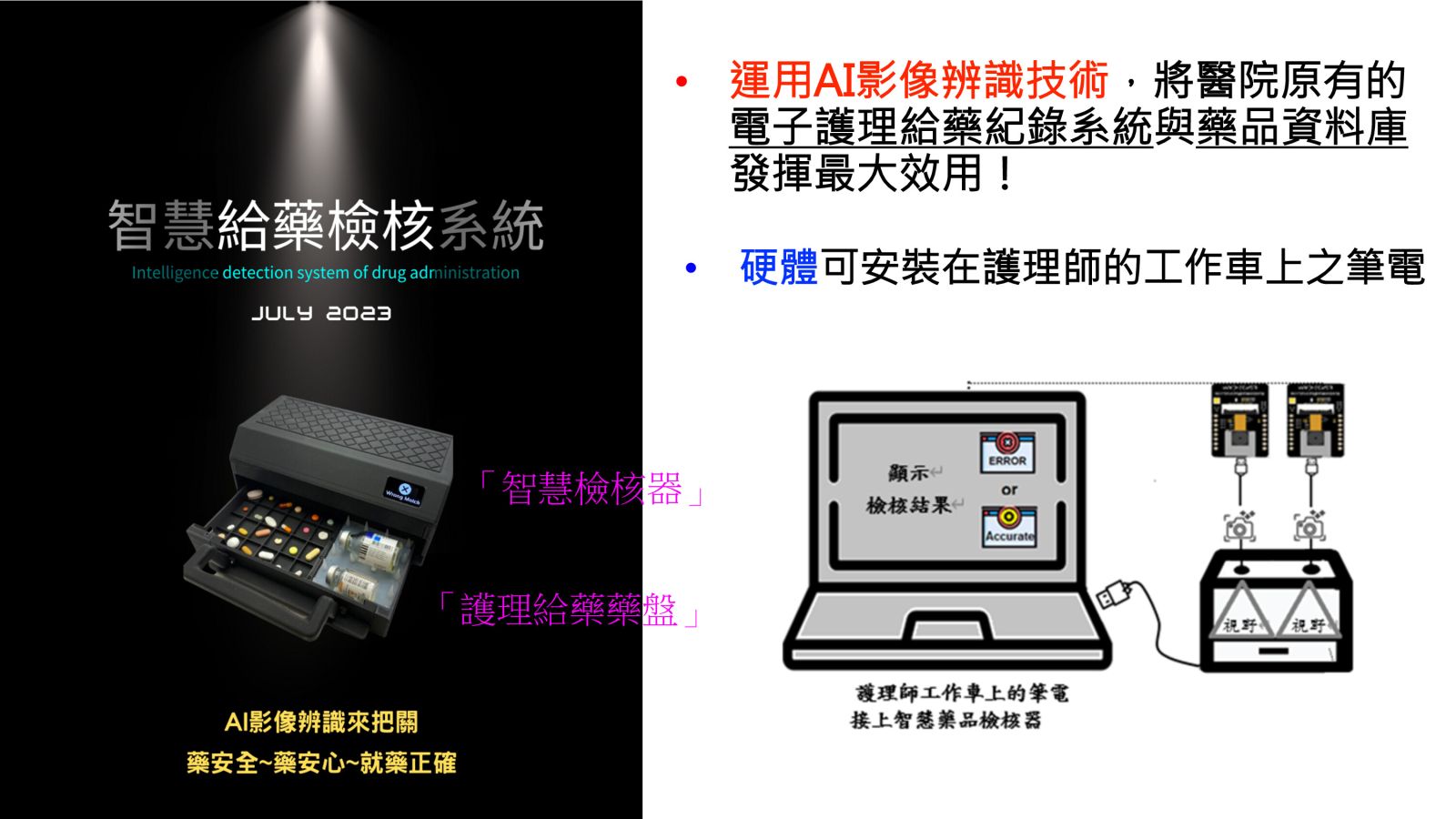
 28智慧護理給藥檢核系統國立臺灣大學護理學系胡文郁教授發明人:李婉菱、朱育瑧、胡文郁、詹魁元、許雅婷、郭冠成、黃嗣棻、莊寶玉、高秀娥、郭哲安、王嬿淇、蕭妃秀 、黃織芬、孫慶耀、陳世英、陳達慶領域:醫療器材適應症:全球有“護理給藥業務需求”之機構,如醫學中心/區域/地區醫院研發階段:醫材雛型開發Prototype development摘要:
28智慧護理給藥檢核系統國立臺灣大學護理學系胡文郁教授發明人:李婉菱、朱育瑧、胡文郁、詹魁元、許雅婷、郭冠成、黃嗣棻、莊寶玉、高秀娥、郭哲安、王嬿淇、蕭妃秀 、黃織芬、孫慶耀、陳世英、陳達慶領域:醫療器材適應症:全球有“護理給藥業務需求”之機構,如醫學中心/區域/地區醫院研發階段:醫材雛型開發Prototype development摘要:美國每年約130萬人因用藥錯誤受到傷害,成本估計為420億美元。在臺灣根據醫策會病人安全通報系統統計,護理給藥階段常見錯誤主要為劑量、頻率、名稱錯誤,是病安重要議題。彙整臺灣近十年護理給藥階段異常件數皆為約5,000件,顯示問題尚未被解決,給藥是護理師最頻繁的業務,僅用人工進行核對藥品,易受多重因素而發生錯誤,故引發本團隊運用AI影像辨識技術,首創「智慧給藥辨識系統」,可檢視護理師「實際拿取的藥物」與「電子醫囑」一致性,若有誤可即時阻止,將可避免大於70%的給藥錯誤類別,且透過應用程式介面(API)可輕鬆串接任一電子給藥系統,是繼2000年美國醫院將條碼導入護理給藥,進行電子檢核的另一新選擇,將可大幅提升病人用藥安全。
In the United States, approximately 1.3 million people are harmed by medication errors each year, resulting in an estimated cost of 42 billion dollars. In Taiwan, according to statistics from the National Health Insurance Administration's Patient Safety Reporting System, common errors during the medication administration phase include dosage errors, frequency errors, and name errors. Patient safety is a crucial concern in this regard.
Over the past decade in Taiwan, there have been approximately 5,000 cases of abnormal incidents during the medication administration phase, indicating that the problem has not been adequately addressed. Medication administration is one of the most frequent tasks performed by nurses, and relying solely on manual verification of medications makes it susceptible to errors due to multiple factors.
Therefore, our team has introduced an " Intelligence detection system of drug administration " using AI image recognition technology. This system allows for the verification of the consistency between the medications a nurse actually dispenses and the electronic prescriptions. If there is an error, it can be immediately prevented. This system has the potential to prevent more than 70% of medication error categories. Furthermore, it can be easily integrated with any electronic medication system through application programming interfaces (APIs).
This innovation is a significant step forward in enhancing patient medication safety, akin to the introduction of barcode scanning for medication administration in US hospitals in the year 2000.-
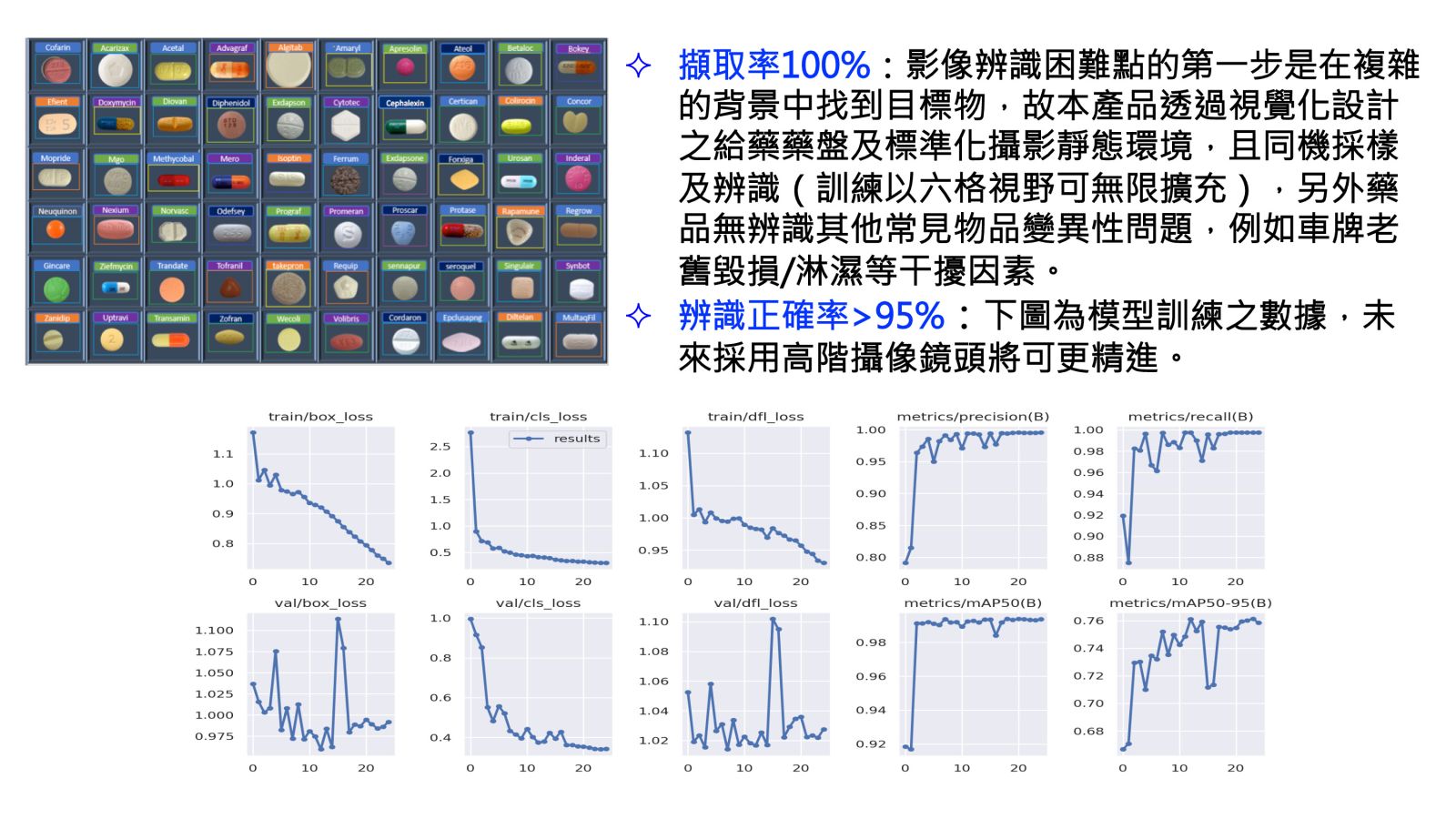
Prototype階段:擷取率達100%以及辨識正確率>95%(附上模型訓練之數據,未來採用高階攝像鏡頭將可更精進)。
 29影像引導氣腹針穿刺術於腹腔鏡手術之氣腹建立國立陽明交通大學郭文娟生醫光電所所長發明人:郭文娟/高孟群/黃逸修領域:醫療器材適應症:腹腔鏡手術之氣腹建立研發階段:2022進行了學研IRB進行10位人體實驗影像收案摘要:
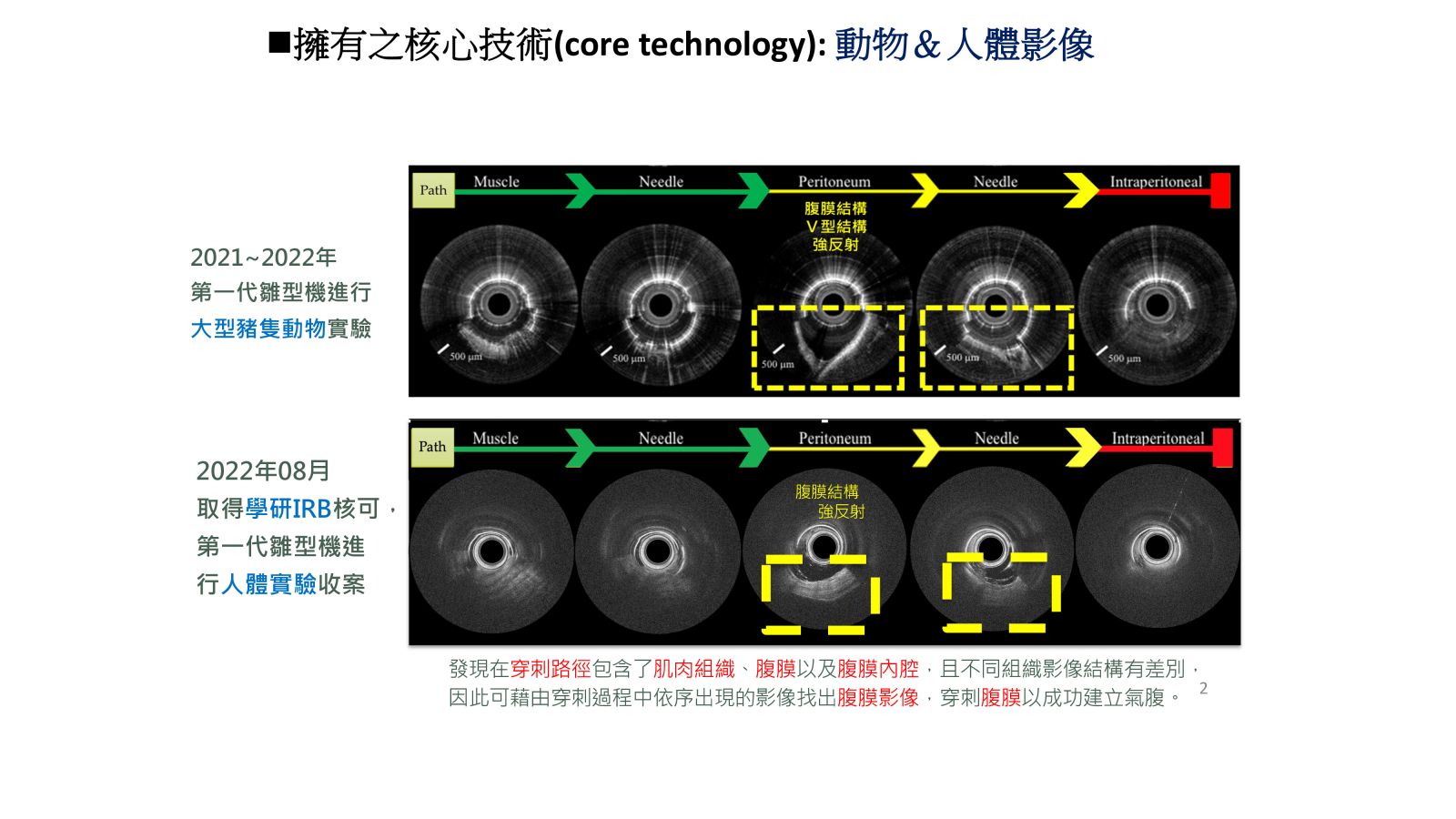
29影像引導氣腹針穿刺術於腹腔鏡手術之氣腹建立國立陽明交通大學郭文娟生醫光電所所長發明人:郭文娟/高孟群/黃逸修領域:醫療器材適應症:腹腔鏡手術之氣腹建立研發階段:2022進行了學研IRB進行10位人體實驗影像收案摘要:腹腔鏡檢查已廣泛應用於許多外科手術,而進入腹腔並形成氣腹,是每個腹腔鏡手術的關鍵第一步。判斷Veress氣腹針是否成功穿刺過腹膜而安全進入腹腔,目前主要仰賴臨床醫師經驗加以判斷,這個作法潛藏風險。在針穿刺腹膜的過程中,盲視插針對於內臟和血管損傷的風險,已知造成至少有一半的主要併發症發生於手術開始之前。這顯示確認氣腹針進入腹腔的步驟,對於成功的腹腔鏡手術至關重要。基於此臨床痛點,我們開發了光學影像引導探針的技術,以即時視別氣腹針的針尖位置。而此技術將氣腹建立由盲刺轉為可視化程序,因而大幅提升氣腹建立的有效及安全性。
Needle puncture is an indispensable technology in clinical medicine, such as in pneumoperitoneum establishment, epidural blockade and fascia plane block procedure, etc. It's very depends on the doctor's experience and touch feel. Our system provides the real-time imaging and automatic classification to guide the needle puncture procedures, thus achieve to precise positioning and required safety. This method will greatly reduce the failure rate of traditional needle punctures and other complications. In the whole-eye OCT positioning system, only one scan is required, and no additional hardware architecture is required to obtain the whole-eye image.
-


提供即時監測,針對病患的個別情形分析來選擇適當的最佳療法
 30利用壓電骨釘治療並預防骨折不癒合國立臺灣大學醫學工程學系王兆麟教授發明人:王兆麟;朱亞成領域:醫療器材適應症:治療並預防骨折不癒合研發階段:動物驗證 In vivo validation摘要:
30利用壓電骨釘治療並預防骨折不癒合國立臺灣大學醫學工程學系王兆麟教授發明人:王兆麟;朱亞成領域:醫療器材適應症:治療並預防骨折不癒合研發階段:動物驗證 In vivo validation摘要:我們的研究顯示,壓電刺激能夠有效增進骨髓幹細胞的遷移、增生與分化,並能幫助軟骨細胞在平面培養環境下維持軟骨特性[3,4]。由於這些生理過程都是骨癒合中重要的元素,因此我們假設壓電刺激可以增進骨癒合,基於這個假設,如果我們能將現行骨折醫材加入壓電材料特性,就能在骨釘植入後不斷對骨折部位進行壓電刺激,藉以促進骨癒合並且避免骨折不癒合的情形出現。我們團隊提出將壓電特性導入目前臨床上使用的骨釘或骨板,讓手術後的骨折部位受到由骨釘所提供的額外壓電刺激,壓電刺激亦可由外部超音波和骨釘的交互作用而產生。此刺激可減短骨癒合時間並減少術後骨折不癒合。
Our research shows that piezoelectric stimulation can effectively enhance the migration, proliferation, and differentiation of bone marrow stem cells and can help maintain the cartilage characteristics of chondrocytes in a culture environment [3,4]. Since these physiological processes are essential elements in bone healing, we hypothesize that piezoelectric stimulation can promote bone healing. Based on this hypothesis, if we can incorporate piezoelectric properties into the current bone fracture implant, we can apply piezoelectric stimulation to the fracture site after implanting, thus promoting bone healing and avoiding nonunion of fractures. Our team proposes to introduce piezoelectric properties into the bone screws or plates currently used in clinical practice, allowing the post-surgery fracture site to receive additional piezoelectric stimulation provided by the implant. If the fracture site cannot be loaded to introduce the piezoelectric stimulations, the piezoelectric stimulation can also be generated by the interaction of external ultrasound and bone screws. This stimulation can shorten the bone healing time and reduce postoperative nonunion of fractures.
-
上:小鼠骨癒合動物實驗的四個階段: 手術、骨裂、植入、術後評估。下:小鼠腿骨術後的強度測試設備(右圖)、第一輪結果(左一圖)和第二輪結果(左二圖),強度測試的結果分三組: 正常腿骨(normal leg)、手術使用金屬骨材(regular pin)、手術使用壓電骨材(PZT pin)。
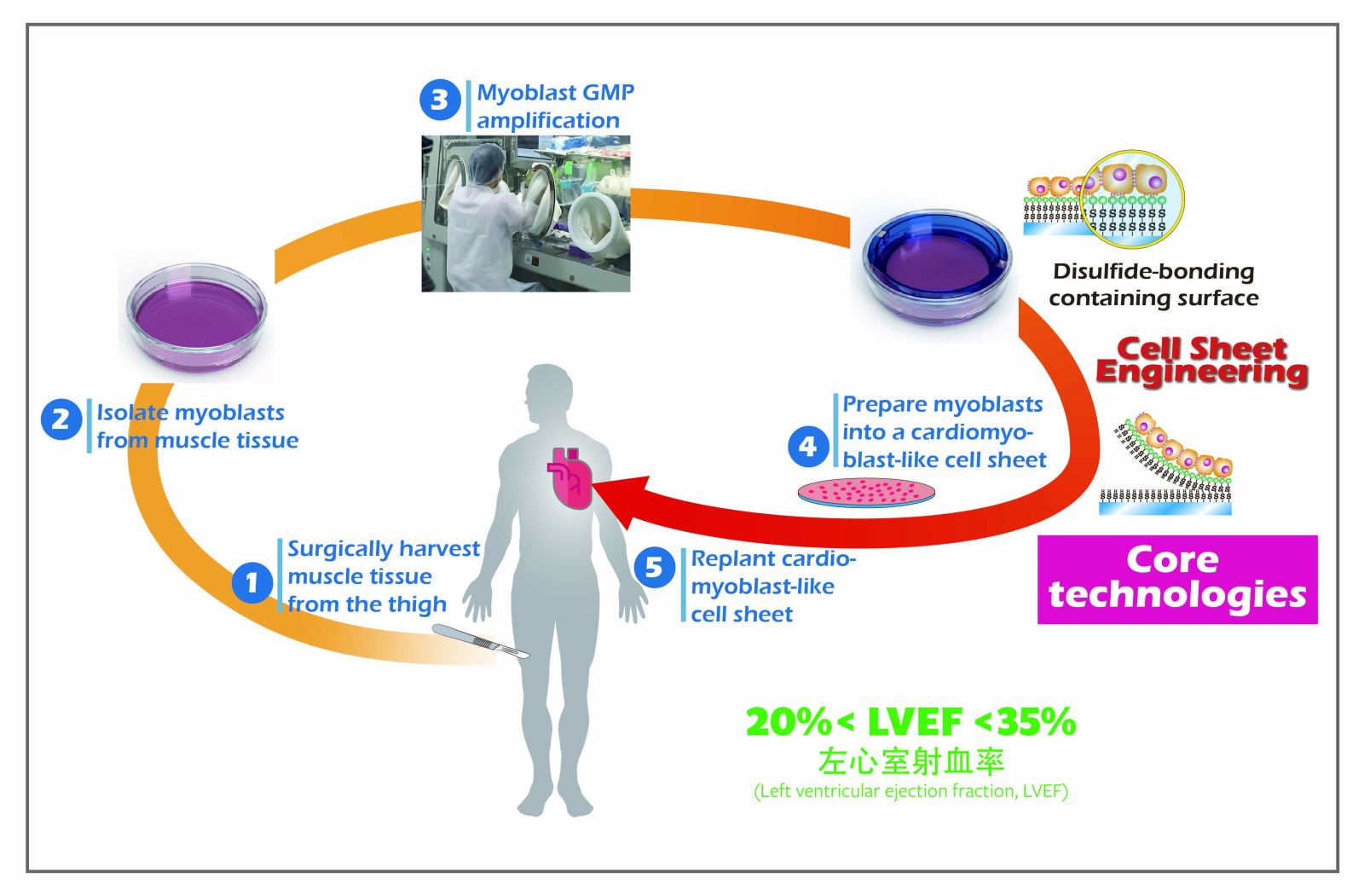
 41片狀細胞工學平台的疾病應用法及其經濟規模臺北醫學大學醫學系曾厚教授發明人:曾厚領域:醫療器材適應症:左心室心肌、子宮內膜、膝軟骨、呼吸道重建研發階段:動物驗證 In vivo validation摘要:
41片狀細胞工學平台的疾病應用法及其經濟規模臺北醫學大學醫學系曾厚教授發明人:曾厚領域:醫療器材適應症:左心室心肌、子宮內膜、膝軟骨、呼吸道重建研發階段:動物驗證 In vivo validation摘要:『將無間質的細胞懸浮液提昇至具間質的組織片,才可展現再生醫療導向細胞治療的潛力。』─是開發片狀細胞工學技術平台最大目的。我們選擇利於細胞的天然高分子與軟硬度製備機能薄膜而能培養出層片組織,此有別傳統使用酵素將細胞之蛋白分解,能保留胞外間質而大幅提高生物利用率。在再生醫學臨床應用上能使用微量自體組織放大成片狀細胞組織,避免排斥反應修復受損部位。我們已成功地應用至角膜上皮、子宮內膜、牙周膜、呼吸上皮、尿道上皮、軟骨、心肌等組織上。再者,本平台的相關技術已獲得多項專利、2次國家新創獎,且部分技術已獲經濟部價值創造計畫的補助,已證明能量產應用。如能商業運轉應能提昇國內再生醫療相關的產業規模並嘉惠病患。
“Upgrading the cell suspension without ECM into a cell sheet with ECM could exhibit the real potential of cell therapy for regenerative medicine. " - This is the major target of developing a cell sheet engineering technical platform. Briefly, amino acids and natural polymers with suitable stiffness and good for cell behaviors were selected to graft into porous membranes and further cultivate/fabricate cell sheets. This is different from the conventional use of enzymes to digest tight junctions. It can retain the ECM and significantly improve bioavailability. In the clinical applications of regenerative medicine, trace amounts of autologous tissue can be applied to amplify into cell sheets to avoid rejection and repair injured tissues. We have successfully applied it to the corneal epithelium, endometrium, periodontal ligament, respiratory epithelium, urothelium, cartilage, esophageal epithelium, or myocardial tissue. Furthermore, the relevant technologies of the platform have obtained multiple patents and 2 National Innovation Awards, and some technologies have received subsidies from the MOEA Technology Development Program for Academia, proving their applications in mass production. If it can be put into commercial operation, it should increase the scale of domestic regenerative medicine-related industries and benefit patients.
-
本圖所示的是治療嚴重缺血性心臟病用的左心室心肌重建整體過程。我們自行發展的新型細胞層片技術具有技術自主性也具有外溢性,開發至今已完成角膜上皮重建用細胞層片的全程製作與驗證,並藉經濟部學界科專計畫技轉至國內生醫公司。而現今最為重要的是驗證適用於治療嚴重缺血性心臟病用的左心室心肌重建,除具醫療需求性亦具市場規模;另,膝蓋軟骨細胞層片也正與國內知名生技公司合作開發中。
 42防導絲滯留之特殊導管接頭設計中央研究院陳常善博士後研究員發明人:陳常善領域:醫療器材適應症:置入靜脈導管造成之傷害研發階段:醫材雛型開發Prototype development摘要:
42防導絲滯留之特殊導管接頭設計中央研究院陳常善博士後研究員發明人:陳常善領域:醫療器材適應症:置入靜脈導管造成之傷害研發階段:醫材雛型開發Prototype development摘要:臨床滯留管路,中央靜脈導管(CVC)、周邊置入中心靜脈導管(PICC)的放置過程中,可能因狀況緊急或人為疏失導致金屬導絲滯留體內的狀況發生,並因此對患者造成嚴重的傷害。本專案在接頭的部分設計特殊構造,協助穩定導絲避免導絲滑入體內,以及增加示警作用提醒操作者移除導絲。本計畫已成功完成工業製圖及3D列印試製測試,並已開模進行塑膠射出試製,完成功能驗證。專利佈局則已提送台灣專利申請,並準備提送美國專利申請中。本計畫目前以授權專利為目標,並保持開放的心態將此案背後所應用之創新醫材設計結構化流程,應用於其他合作關係。
 43個人化免疫力評估監控平台臺北醫學大學藥學院臨床藥學科張偉嶠教授發明人:張偉嶠領域:醫療器材適應症:癌症、感染性疾病、自體免疫疾病、移植、疫苗。研發階段:臨床檢體/細胞驗證 Clinical specimen/In vitro validation摘要:
43個人化免疫力評估監控平台臺北醫學大學藥學院臨床藥學科張偉嶠教授發明人:張偉嶠領域:醫療器材適應症:癌症、感染性疾病、自體免疫疾病、移植、疫苗。研發階段:臨床檢體/細胞驗證 Clinical specimen/In vitro validation摘要:免疫力是最好的老師,免疫的辨識與波動可以即時反映一個人的健康狀態。如何科學化的量測免疫力呢?最好的做法就是利用高通量定序的方式,分析T細胞及B細胞的受體重組多樣性,並將免疫細胞鑑別抗原的序列分析出來。我們提供快速且經濟實惠的客製化免疫組建庫試劑及分析服務,可以協助生技公司在疫苗開發之臨床前開發動物實驗免疫原性分析,及臨床試驗階段的免疫反應監控。
Immunity is the best teacher; the immune recognition and fluctuations can instantly reflect a person's health status. How can we scientifically measure immunity? The best approach is to use high-throughput sequencing to analyze the repertoire diversity of T cells and B cells and to identify the antigen sequences recognized by immune cells. We provide fast and cost-effective immune repertoire library reagents and analysis services to assist biotech companies in pre-clinical development of immunogenicity analysis for vaccine development in animal experiments and monitoring immune responses during human clinical trials.
-
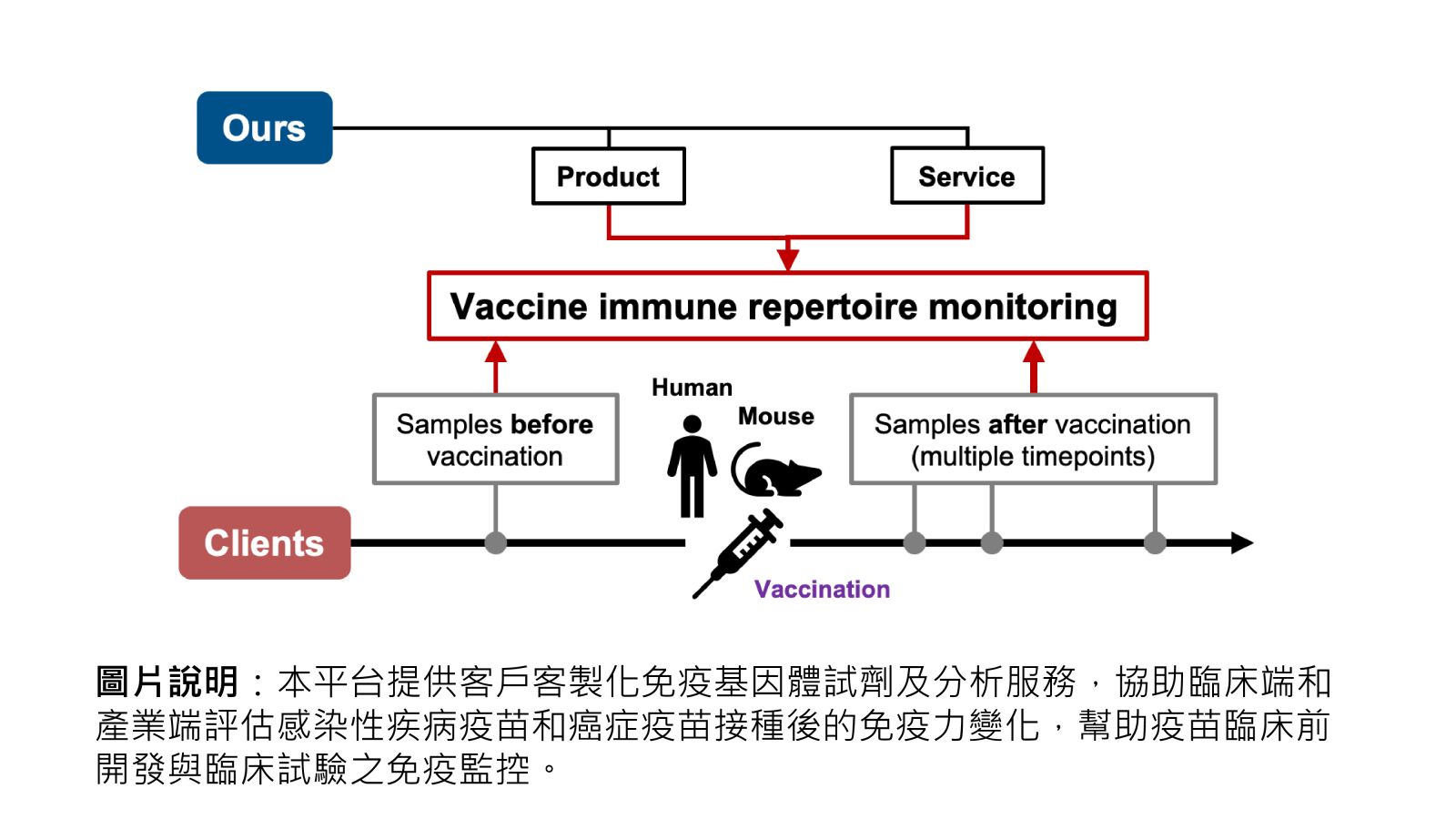
圖片說明:本平台提供客戶客製化免疫基因體試劑及分析服務,協助臨床端和產業端評估感染性疾病疫苗和癌症疫苗接種後的免疫力變化,幫助疫苗臨床前開發與臨床試驗之免疫監控。
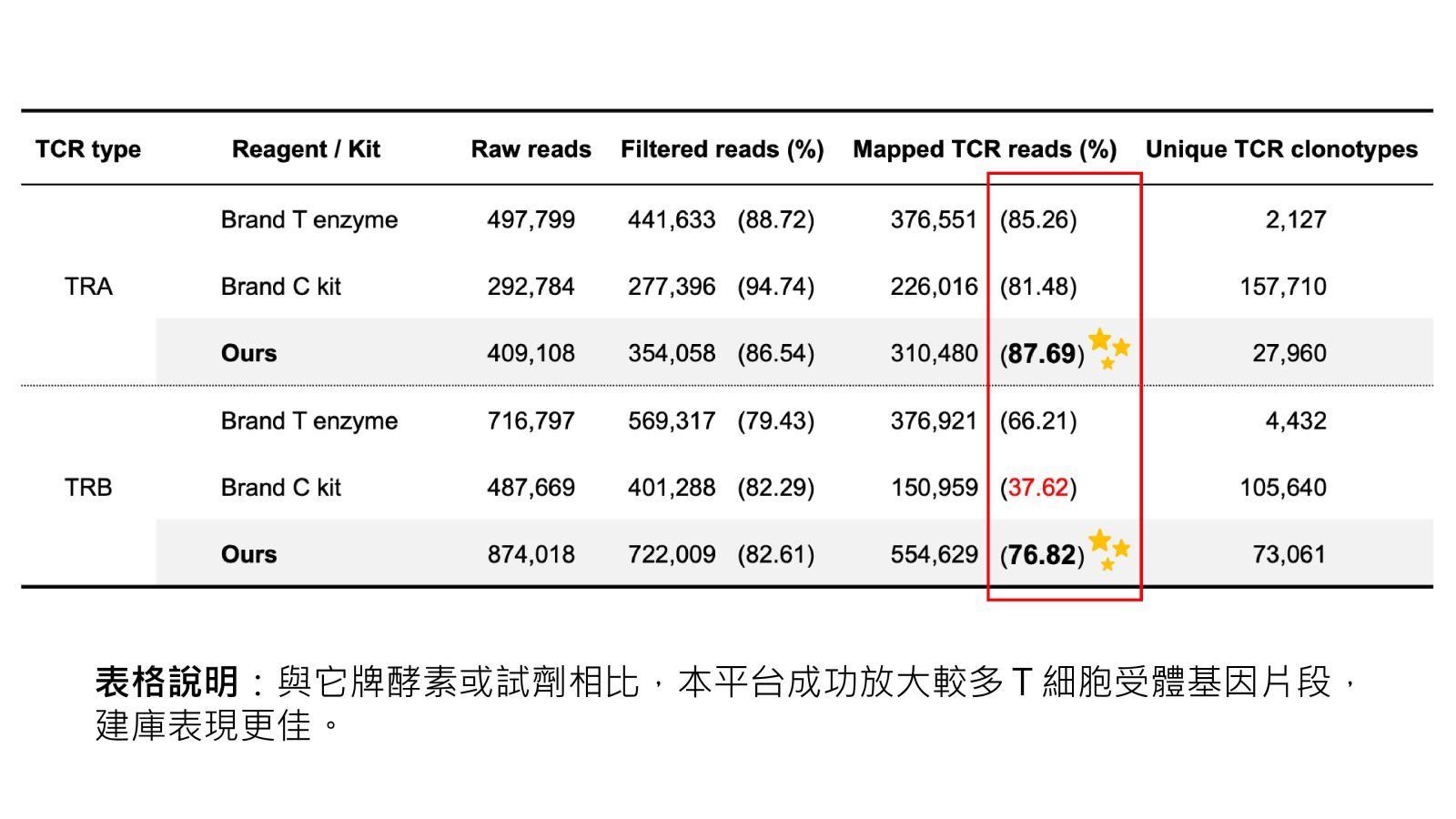
表格說明:與它牌酵素或試劑相比,本平台成功放大較多T細胞受體基因片段,建庫表現更佳。 44次世代細胞治療品質監控平台國立陽明交通大學柯泰名/柯宜芬 副教授發明人:柯泰名 楊裕雄 陳冠行 林哲民 柯宜芬領域:醫療器材適應症:癌症細胞治療 / Cancer Cell therapy研發階段:醫材雛型開發Prototype development摘要:
44次世代細胞治療品質監控平台國立陽明交通大學柯泰名/柯宜芬 副教授發明人:柯泰名 楊裕雄 陳冠行 林哲民 柯宜芬領域:醫療器材適應症:癌症細胞治療 / Cancer Cell therapy研發階段:醫材雛型開發Prototype development摘要:「下一代細胞治療監控與品質保證」為CAR-T細胞療法提供了突破性的方法,使用患者自己的T細胞來增強其對抗癌症的能力,同時減少可能的治療排斥。標準程序涉及使用MACS進行細胞排序,然後使用Flow和PCR進行產品特性的測試,接著進行無菌、黴菌和內毒素測試,然後再給予CAR-T細胞。臨床設置中存在即時監測CAR-T細胞的重要缺口。我們這次所提出的晶片技術解決了此問題,可以在各個階段監測CAR-T細胞,確保迅速的質量控制。為了簡化R&D和成本效益,生物製劑公司越來越依賴CDMO進行端到端的開發。成功商業化的自體細胞療法需要製造商、運輸服務和醫院之間的協同合作。所提出的技術確保生產、運輸和應用中的均勻品質,增強了商業可行性。市場預測顯示,細胞和基因治療(CGT)的CDMO市場將從2020年的20億美元增長到2026年的101億美元,細胞療法的份額將變得更加突出。此外,增強個性化醫療治療的伴侶診斷(CDx)市場預計將從2022年的64億美元增長到2029年的135.7億美元。此外,對於各種生物醫學應用至關重要的全球生物芯片市場,預計將從2022年的95.1億美元增長到2030年的259.2億美元,其中北美在市場份額中處於領先地位,亞太地區將見證迅速增長。
"Next-Gen Cell Therapy Monitoring and Quality Assurance" offers a groundbreaking approach to CAR-T cell therapy, enhancing a patient's ability to combat cancer by using their own T cells, while mitigating potential treatment rejections. Standard procedures involve cell sorting with MACS, followed by Flow and PCR testing for product characterization, and subsequent sterility, mycoplasma, and endotoxin tests before administering CAR-T cells. A critical gap in real-time CAR-T cell monitoring exists in clinical settings. The proposed silicon wafer technology addresses this by monitoring CAR-T cells at various stages, ensuring rapid quality control. To streamline R&D and cost-efficiency, biologics firms are increasingly relying on CDMOs for end-to-end development. Successful commercialization of autologous cell therapies necessitates a synergistic collaboration between manufacturers, transport services, and hospitals. The technology presented ensures uniform quality across production, transport, and application, bolstering commercial viability. Market forecasts suggest a booming CDMO market in cell and gene therapy (CGT), projected to rise from $2 billion in 2020 to $10.1 billion by 2026, with cell therapy's share becoming more prominent. Additionally, the Companion Diagnostics (CDx) market, which enhances personalized medical treatments, is anticipated to grow from $6.4 billion in 2022 to $13.57 billion by 2029. Moreover, the global biochip market, integral for various biomedical applications, is set to surge from $9.51 billion in 2022 to $25.92 billion by 2030, with North America leading in market share and Asia-Pacific witnessing rapid growth.
.jpg)
提供即時監測,針對病患的個別情形分析來選擇適當的最佳療法
 45腹腔鏡無網片式疝氣針嘉義長庚紀念醫院小兒外科王世憲主治醫師發明人:王世憲領域:醫療器材適應症:腹股溝疝氣研發階段:醫材雛型開發Prototype development摘要:
45腹腔鏡無網片式疝氣針嘉義長庚紀念醫院小兒外科王世憲主治醫師發明人:王世憲領域:醫療器材適應症:腹股溝疝氣研發階段:醫材雛型開發Prototype development摘要:利用單孔腹腔鏡內環縫合術(Percutaneous internal ring suture, PIRS)治療兒童間接型腹股溝疝氣已有20年的歷史。在偶然的機會裡我們發現成人的間接型疝氣也適合用這種方式修補。進一步查閱文獻發現,有高達7成的成人疝氣也是屬於間接型。經過這幾年不斷的努力,我們不但一一解決了在執行成人PIRS手術過程中會遭遇到的種種問題,也率先發展出”確認類型再開刀”的疝氣精準醫療模式。本腹腔鏡疝氣針組團隊自去年112年8月成軍以來,先後完成了1.針組雛型品的製備,,並開始進入法規試驗及相關動物實驗。 2.確認本產品軟組織修補針組的可專利性。目前正進行下一步的專利佈局,並繼續往商品化的方向努力。未來希望有機會將這個精準醫療的概念導入疝氣醫學的領域,而本軟組織修補針組則有機會改變目前成人疝氣手術一律要補上人工網膜的現況。
The technique of percutaneous internal ring suture (PIRS) has been used for the treatment of pediatric indirect inguinal hernia for over two decades. Incidentally, we found that the technique of PIRS was also feasible for the repair of adult indirect inguinal hernia. Reviewing the literature, as high as 70% of the adult inguinal hernias belong to indirect type. In the past few years, we have resolved all the problems that encountered while applying the technique of PIRS in repairing the adult inguinal hernias. We also contributed to precision medicine in the field of hernia repair and presented the innovative idea that “repair the inguinal hernia according to their type”. Our team for developing the “Percutaneous Internal Ring Ligation Set” was established in August, 2022. Now we have got some achievements: First, we have made the prototypes of the ligation needle set and continue for the execution of the following regulatory certifications and animal experiments. Second, we have completed the analysis of the patentability of this innovative soft-tissue repair set. The layout for the patent portfolio and commercialization are keep going. It is possible that our novel precision medicine idea and the soft-tissue repair needle set may change the concept that every kind of adult inguinal hernia needs to receive an artificial mesh repair.
-
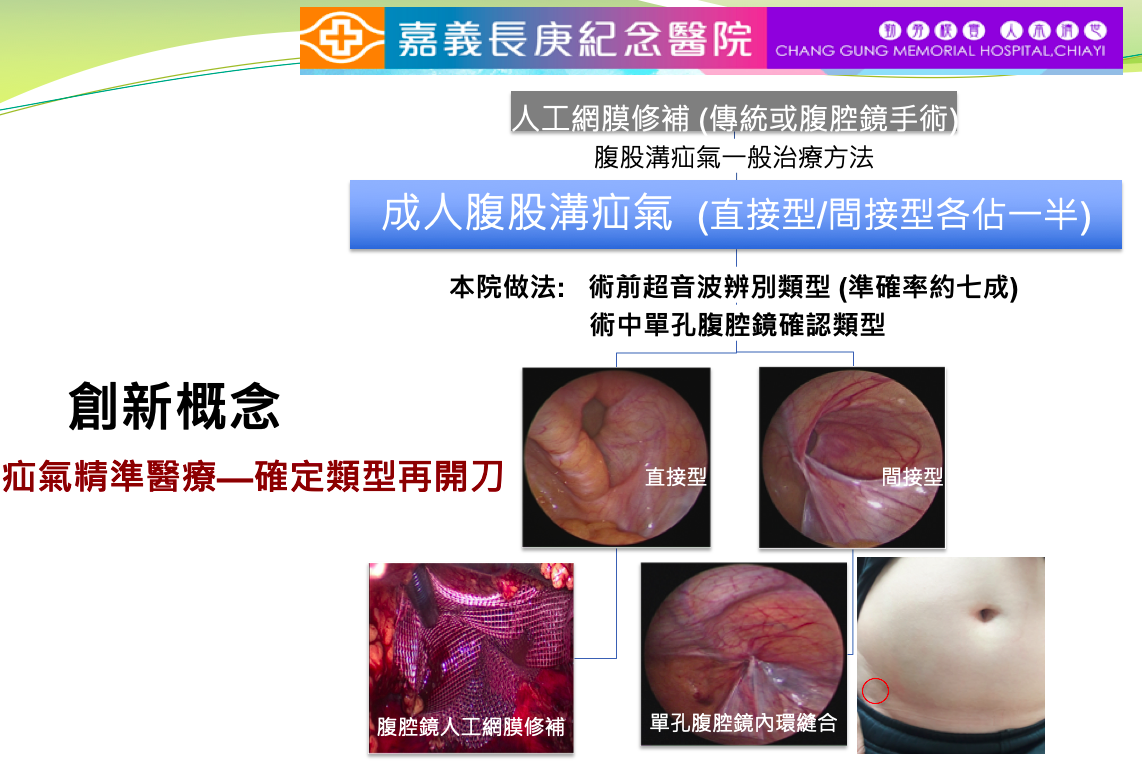
目前疝氣治療的觀念是一律要補上人工網膜(不論是傳統或微創手術),本團隊率先發展出根據類型做修補的創新概念,特別針對間接型疝氣的修補我們設計出"無網片式疝氣針",可讓絕大部份疝氣患者不必補上人工網片,手術時間短,術後不痛,免住院,並迅速恢復日常.
 46細胞基因體數據驅動之新興與未知感染源藥物標靶快速鑑定平台國立陽明交通大學 /中央研究院生物醫學科學研究所柯泰名副教授 / 林哲民合聘助研究員發明人:柯泰名 簡文森 張兆良 林芳平 林哲民領域:醫療器材適應症:小兒血管炎研發階段:活性化合物/生物標誌確認 Hit compounds/biomarkers confirmation摘要:
46細胞基因體數據驅動之新興與未知感染源藥物標靶快速鑑定平台國立陽明交通大學 /中央研究院生物醫學科學研究所柯泰名副教授 / 林哲民合聘助研究員發明人:柯泰名 簡文森 張兆良 林芳平 林哲民領域:醫療器材適應症:小兒血管炎研發階段:活性化合物/生物標誌確認 Hit compounds/biomarkers confirmation摘要:全球醫療保健產業正在努力應對發炎症候群和不明傳染原(例如川崎病 (KD) 和兒童多系統發炎症候群 (MIS-C))的挑戰。 儘管這些綜合徵有相似之處,但需要大量資源進行診斷和治療。迅速介入的緊迫性、有效療法的缺乏以及對綜合治療方法的需求加劇了醫療保健困境。為了滿足這些需求,引入了突破性的轉化醫學管道,旨在快速識別藥物標靶。該解決方案利用單細胞基因組數據,融合生物資訊學、系統生物學和化學資訊學,從而加快藥物發現過程,並有可能在細胞層面上解決病症。它的多功能性延伸到許多疾病,表明其具有廣泛的治療影響。該領域的趨勢強調大數據和計算方法在研發中的使用、公共單細胞數據的利用以及個人化醫療的發展。該技術因其在細胞層面逆轉疾病的潛力而脫穎而出,使其成為該領域無與倫比的解決方案。隨著全球健康危機凸顯了做好準備的必要性,該技術與利用數據分析和體學數據快速做出反應的推動相一致。 台灣以其一流的醫療保健而聞名,國內對此類先進解決方案的需求強勁。 市場預測表明,單細胞技術目前的價值為24 億美元,到2032 年可能會飆升至155 億美元。此外,計算生物學預計到2030 年將從29.6 億美元增長到令人印象深刻的348.7 億美元。具有成本效益的研發模式,承諾更快地進行藥物再利用和發現,並有能力提供整體治療解決方案。
The global healthcare sector grapples with challenges posed by inflammatory syndromes and unidentified infectious agents, such as Kawasaki Disease (KD) and Multisystem Inflammatory Syndrome in Children (MIS-C). These syndromes, despite their similarities, necessitate extensive resources for diagnosis and treatment. The urgency for swift intervention, absence of effective therapies, and the need for a comprehensive treatment approach exacerbate the healthcare dilemma. In addressing these needs, a groundbreaking translational medicine pipeline has been introduced, designed for rapid drug target identification. This solution harnesses single-cell genomic data, fusing bioinformatics, systems biology, and cheminformatics, thus expediting the drug discovery process with the potential to address conditions at the cellular level. Its versatility extends to numerous diseases, indicating a broad therapeutic impact. Trends in this domain emphasize the use of big data and computational methods for R&D, public single-cell data utilization, and the movement toward personalized medicine. The technology stands out for its potential to reverse diseases at the cellular level, making it an unparalleled solution in the field. With global health crises underscoring the need for preparedness, the technique aligns with the push for swift responses using data analytics and omics data. Taiwan, known for its stellar healthcare, presents a strong domestic demand for such advanced solutions. Market projections indicate that single-cell technologies, currently valued at USD 2.4 billion, could surge to USD 15.5 billion by 2032. Additionally, computational biology is slated to grow from USD 2.96 billion to an impressive USD 34.87 billion by 2030. This technology offers a cost-effective R&D model, promising faster timelines for drug repurposing and discovery, with the capability to provide holistic therapeutic solutions.
-
A. The computational workflow involving single-cell meta-analysis, bioinformatics, and cheminformatics techniques. B. Schematic representation of the translational informatics approach in application to pediatric hyperinflammatory syndromes, KD and MIS-C.
 47Constructing and Prototyping 3D Functional Scaffolds Using Novel in-House-Developed Autopilot Single-Jet Electrospinning Platform for Tissue Engineering and Regenerative Medicine Applications中央研究院物理研究所周家復特聘研究員發明人:Chia-Fu Chou, Balchandar Navaneethan領域:醫療器材適應症:Tissue Engineering and Regenerative Medicine研發階段:醫材雛型開發Prototype development摘要:
47Constructing and Prototyping 3D Functional Scaffolds Using Novel in-House-Developed Autopilot Single-Jet Electrospinning Platform for Tissue Engineering and Regenerative Medicine Applications中央研究院物理研究所周家復特聘研究員發明人:Chia-Fu Chou, Balchandar Navaneethan領域:醫療器材適應症:Tissue Engineering and Regenerative Medicine研發階段:醫材雛型開發Prototype development摘要:迄今為止,在臨床治療中以人造組織結構來替代傳統的組織移植物極具挑戰性。人體組織的多 尺度複雜性考驗了現有方法(包括技術先進的 3D 列印)的有效性,由於其不易實現組織工程 和再生醫學 (TERM) 應用的臨床適用結構。 我們最近報導了一種新穎的自駕單射流 3D 電紡 絲工藝。該工藝能夠通過自我複制從小到大尺寸的 3D 模板(具簡單到複雜的幾何形狀)來自 主構建 3D 形廓支架,類似蠶繭結構,並採用 FDA 核准的可生物降解聚合物聚己內酯 (PCL) 的單射流,製作具有高解析度和形狀保真度的細胞支架。獨特的單射流在微懸臂式射流 (M1) 3 和鞭打射流 (M2) 之間的自動切換以及獨特的環場感測及高目標特異性,使電紡絲能有序且自 排列的製作仿天然細胞外基質結構,並共形地貼附在 2D 和 3D 模板上,以生成模仿人體器官的 3D 形廓支架,例如 3D 人體面部、血管、乳房和乳頭,均具有梯度孔隙率和強大的力學性能, 可自站立並有高度的形狀記憶性。值得注意的是,單射流 3D 電紡的自構建功能強大、再現性 高且具有高靈活性,從而能構建各種二維及三維支架,可廣泛的應用於 TERM。在成功完成原 型機和自動化後,我們計劃將推出第一個商用 3D 電紡絲平台至價值數十億美元的 TERM 市 場,並希望在未來 3 年內成立新創公司及展示數項 TERM 的應用。
To this date, engineering artificial tissue constructs to replace the traditional tissue grafts in the clinical treatments is extremely challenging. The multiscale complexities of human tissues highly contest the effectiveness of the existing approaches, including the technically-advanced 3D printers, therefore preventing them from achieving clinically applicable constructs for tissue engineering and regenerative medicine (TERM) applications. We have recently reported a novel Autopilot Single-Jet (AJ) 3D Electrospinning (ES) process, which is capable of self-constructing 3D topographic scaffolds via self-replicating small- to large-scale 3D templates with simple to complex geometries, similar to silkworm cocoon construction, with high-resolution and shape-fidelity using the FDA-approved Polycaprolactone (PCL) biodegradable polymer single jet. The unique auto-jet switching of the single jet between novel microcantilever-like armed jet (M1) and whipping jet (M2) and exclusive 360⁰ field sensing together with high-target specificity have orderly, yet self-arranged the native extracellular matrix mimicking electrospun fibers conformally on both the 2D and 3D templates to produce human-organ-mimicking 3D topographic scaffolds, e.g. human 3D face, blood vessel, female breast & nipple, with gradient porosity and robust mechanical properties, e.g., free-standing with excellent shape memory. Remarkably, the self-construction of the AJ-3D ES is robust, highly reproducible, and with a high degree of flexibility thus allowing it to construct varieties of 2D and 3D scaffolds for a wide range of TERM applications. Upon successful prototyping and automation, we plan to introduce the first commercial 3D electrospinning platform to the multibillion dollars’ TERM market with a company spin-off and a few applications deliverable within the next 3 years.
-
Work flow of proposed ideas. (1) components of AJ-3D ES platform to be prototyped. (2) 4-step work flow for the construction of 3D topographic fibrous scaffolds. (3) Proposed applications of 3D topographic fibrous scaffolds.
